“iPSC Characterization Kit Market” in terms of revenue was poised to grow at a CAGR of 15.54% from 2024 to 2031 according to a new report by Insight Ace Analytic.
Request for Sample Pages: https://www.insightaceanalytic.com/request-sample/1418
Latest Drivers Restraint and Opportunities Market Snapshot:
Key factors influencing the global iPSC Characterization Kit market are:
- Rising Prevalence of Chronic Diseases
- Growing Applications in Research and Therapy
- Increasing Research Funding
The following are the primary obstacles to the iPSC Characterization Kit market’s expansion:
- Ethical and Regulatory Challenges
- High Cost Associated with Stem Cell Therapy
- Availability of Alternatives for Tumor Treatments
Future expansion opportunities for the global iPSC Characterization Kit market include:
- Expansion of Therapeutic Applications
- Advanced Characterization Kit Technologies
- Expansion of Markets by Market Players
Market Analysis:
The increasing applications in disease modeling, drug discovery, and regenerative medicine have created a high demand for reliable and efficient iPSC characterization tools. That addresses the need by providing comprehensive and standardized assays, saving researchers time and effort in evaluating and characterizing their iPSCs. Furthermore, the increasing focus on personalized medicine and the development of targeted therapies is expected to boost the demand for iPSCs and their characterization kits further.
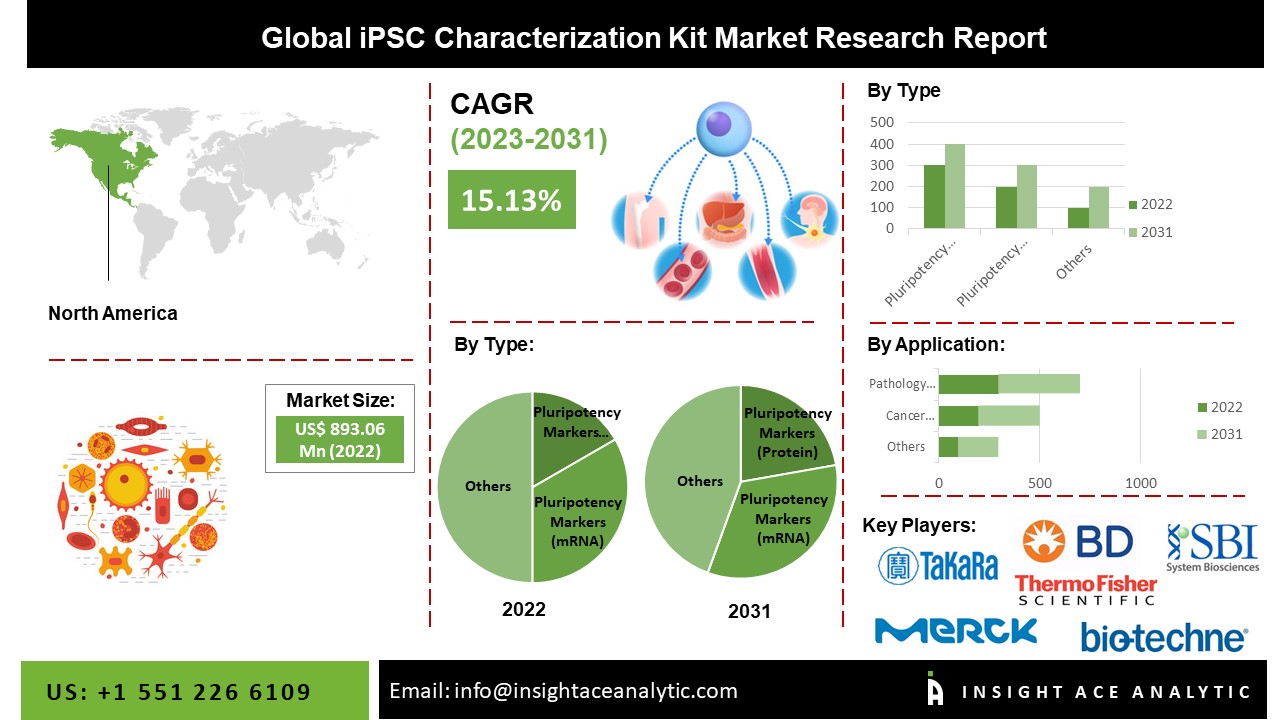
List of Prominent Players in the iPSC Characterization Kit Market:
- Takara Bio
- Thermo Fisher Scientific
- BD Biosciences
- Applied StemCell
- Amsbio
- Bio-Techne
- ALSTEM
- STEMCELL Technologies
- System Biosciences
- Applied Biological Materials
- Creative Bioarray
- Elixirgen Scientific
- Miltenyi Biotec
- Merck KGaA
iPSC Characterization Kit Market Report Scope:
|
Report Attribute |
Specifications |
|
Growth rate CAGR |
CAGR of 15.54% from 2024 to 2031 |
|
Quantitative units |
Representation of revenue in US$ Million, and CAGR from 2024 to 2031 |
|
Historic Year |
2019 to 2023 |
|
Forecast Year |
2024-2031 |
|
Report coverage |
The forecast of revenue, the position of the company, the competitive market structure, growth prospects, and trends |
|
Segments covered |
By Product, Patient Type, End-Use |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
Recent Developments:
- In August 2021, Fate Therapeutics officially declared that FT819 had been successful in treating its first patient in a clinical trial. FT819, which is a CAR-T cell treatment, was made utilizing iPSCs.
- In May 2022, Sernova and Evotec discussed a strategic treaty to develop an iPSC-based beta substitute therapy for type 1 and 2 diabetes.
- In July 2021, SCM Lifescience Co. Ltd., a South Korea-based cell therapy developing firm, pronounced that it had in-license a diabetes drug from Allele Biotechnology and Pharmaceuticals, Inc., which is a pancreatic beta cell therapy extracted from iPSCs, and the deal has been signed at a valuation of US$ 0.75 million.
Curious about this latest version of the report? @ https://www.insightaceanalytic.com/enquiry-before-buying/1418
iPSC Characterization Kit Market Dynamics:
Market Drivers: Growing Applications in Research and Therapy
The increasing use of applications in research and therapeutic developments drives the global market. iPSCs are being used as versatile tools for studying disease mechanisms, drug discovery, and personalized medicine for research, which enables the investigation of disease progression, identification of therapeutic targets, and screening of candidate drugs using iPSC-derived cell models. Furthermore, iPSCs hold immense promise in therapeutic development for regenerative medicine and cell-based therapies that offer novel treatment strategies for diseases and injuries, including neurodegenerative disorders, cardiovascular diseases, and musculoskeletal injuries. Moreover, the characterization kits tailored for evaluating the quality of iPSC-derived cell therapies are in high demand as these therapies advance through preclinical and clinical development stages, thereby accelerating progress in stem cell research and therapeutic translation.
Challenges: High Cost Associated with Stem Cell Therapy
The huge cost associated with stem cell therapy poses restraint by limiting patient access and hindering stem cell-based treatments’ commercial viability and scalability. The complex manufacturing processes necessary to culture, expand, and differentiate stem cells into therapeutic cell types incur significant expenses related to specialized equipment, reagents, and skilled personnel. Furthermore, expenses associated with conducting clinical trials, monitoring, patient recruitment, and regulatory submissions are considerable. Limited reimbursement coverage for stem cell therapies in many healthcare systems exacerbates the affordability challenge, restricting patient access and hindering stem cell-based treatments’ commercial viability and scalability.
North America Is Expected To Grow With the Highest CAGR during the Forecast Period
The North American iPSC Characterization Kit Market is expected to record a significant revenue share and develop at a rapid CAGR soon. It is because this region is the hub for biomedical research and development, with renowned institutions and biotechnology companies that drive innovation in stem cell research. This biotechnology industry generates a high demand for iPSC characterization kits to support research activities. Furthermore, the region’s well-established healthcare infrastructure and extensive network of research institutions facilitate market awareness and adoption of iPSC characterization kits across various applications, including drug discovery and regenerative medicine. Collectively, these factors contribute to North America’s prominent position in the iPSC characterization kit market.
Segmentation of iPSC Characterization Kit Market-
By Type-
- Alkaline Phosphatase Staining Assay
- Pluripotency Markers (Protein)
- Pluripotency Markers (mRNA)
By Application-
- Cancer Research Center
- Pathology Laboratory
- Academic and Research
- Contract Research Organizations
- Others
By Region-
North America-
- The US
- Canada
- Mexico
Europe-
- Germany
- The UK
- France
- Italy
- Spain
- Rest of Europe
Asia-Pacific-
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
Latin America-
- Brazil
- Argentina
- Rest of Latin America
Middle East & Africa-
- GCC Countries
- South Africa
- Rest of Middle East and Africa
For More Customization @ https://www.insightaceanalytic.com/customisation/1418
Media Contact
Company Name: InsightAce Analytic Pvt. Ltd
Contact Person: Diana D’Souza
Email: Send Email
Country: United States
Website: https://www.insightaceanalytic.com/